Clinical Trials at the Department of Urology
Clinical trials are the most important tool for testing new medical procedures and evaluating their effectiveness and safety.
Doctors and researchers are constantly seeking ways to improve medical treatments for patients.
To advance scientific knowledge, medical professionals design study projects—known as clinical trials—in which volunteer participants can take part.
Most clinical trials focus on the experimental testing of new treatment methods. Study physicians assess whether these treatments are safe, effective, and possibly better than existing standard therapies. Under clearly defined conditions, clinical trials may test new medications and therapies, new combinations of existing treatments, or innovative approaches to radiation therapy or surgery.
Participants in clinical trials are often among the first to receive promising new treatments—well before they become available to the general public.
However, there is no guarantee that the new treatment will prove to be safe, more effective, or more reliable than the current standard.
There are also clinical trials aimed at exploring new ways to reduce symptoms or side effects during treatment, as well as long-term effects afterward. Please speak with your doctor for more information.
In addition, ongoing research is being conducted to develop preventive strategies to reduce the risk of disease. Yesterday’s research leads to today’s treatments and tomorrow’s cures.
- What do trials accomplish?
| Trials test new ways to | Prevent bladder cancer |
| Diagnose bladder cancer | |
| Treat or cure bladder cancer | |
| Improve quality of life during and after treatment |
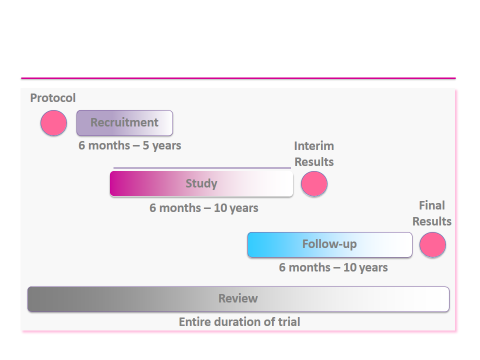
- What do clinical trial “phases” mean?
Each clinical trial is classified into one of 4 phases, depending on what it is testing:
| Phase 1 | Phase 2 | Phase 3 | Phase 4 |
| Ensuring safety | Further evaluating safety | Confirming effectiveness | Monitoring long term effects |
| Identifying side effects | Testing effectiveness of a treatment | Comparing to current standard treatments | Identifying new uses of the treatment |
| Determining safe dosage | Monitoring side effects |
- What happens next?
| Potentially eligible patients are identified |
| They receive detailed information from their doctors about the trial |
| They are asked to sign an “Informed Consent” form to show that they have been properly informed about the study |
- How are patients recruited?
| Patients are chosen based on factors including age, gender, type and stage of bladder cancer, treatment history and other medical conditions |
Sometimes patients must be recruited from many countries in order to ensure that enough people will participate so that the study will have statistical validity |
- Do all patients receive the same treatment?
| All Phase 3 trials (and some in Phase 2) use randomization by computer to put patients into groups receiving different treatments |
| One group (the control group) receives the current standard of care |
| One or more groups receive the therapy being tested, sometimes in different combinations or schedules |
- How long does a trial last?
| Researchers monitor patients for weeks, months or years, depending on the trial protocol |
| Some trials follow patients for more than 10 years, for example to track long-term safety |
- Risks include:
Experimental treatment could prove ineffective |
Known and unknown treatment side effects |
Additional time required at the hospital for tests and check-ups |
- Benefits include:
Access to treatments under development not available outside the trial |
Close medical attention, including additional tests and check-ups |
Contribution to potentially improved treatments for future patients |
- Why support clinical trials?
| Clinical trials provide us with the knowledge we need to treat bladder cancer better |
Now is the time for scientists to make significant progress in answering questions about how to treat and cure bladder cancer |
Support trials and enable progress towards a cure for bladder cancer |
Candidates for a clinical trial choose to participate for various reasons.
For some patients, a clinical trial may represent the best available treatment option. Since standard therapies do not always offer guaranteed success, some patients are willing to accept the added uncertainty in the hope of a better outcome. Others join clinical trials with the understanding that these studies may be the only way to achieve progress in treating diseases like theirs. Even if they do not benefit directly, their participation may help future patients.
Some participants may worry that they will receive only a placebo instead of an actual treatment. However, placebos are usually combined with standard therapy in clinical trials. If a placebo is used in a study, patients are fully informed beforehand and still receive the known standard treatment.
To participate in a clinical trial, patients or candidates must undergo an informed consent process and sign a consent form.
During this conversation, the physician should clearly explain all available options and help the patient understand how the new treatment differs from the current standard treatment. The physician must also explain any potential risks associated with the new therapy, including those that may differ from the risks of standard care.
Patients will also be informed about the inclusion and exclusion criteria for the study, the required follow-up appointments and examinations, and the treatment planParticipation in a clinical trial is entirely voluntary, and patients can withdraw at any time—either for personal or medical reasons. Possible reasons for withdrawal include lack of response to the treatment or the occurrence of serious side effects. All clinical trials are closely monitored by experts for safety and potential complications. Please discuss everything thoroughly with your doctor.
Scientific research through clinical trials is continuously conducted at our clinic for all types of urological diseases.
You can learn more about the current clinical trials in the "Clinical Trials" section.